Cosmetic Product Information Document (PIF)
A product information file is a cosmetic file that contains all the most important information about the finished product, the ingredients of the product, the packaging of the product for sale, the manufacturing process and the product label, which shows the characteristics, safety and effectiveness of the product. A product information document is required for each product placed on the EU/UK market. 
In accordance with EU Cosmetics Regulation 1223/2009(same as UK Cosmetics Regulation), the responsible person must keep a product information file for each EU/UK market cosmetics at the address specified on the product label, which is readily accessible to the competent authority of the EU Member State (or UK) established by the responsible person. The product information document must be in a language easily understood by the local authority in the country responsible for keeping the cosmetic product information document.
When cosmetics are put on the market, the person in charge shall keep product information files for them. Product information files shall be kept for 10 years from the date of the last batch of cosmetics being put on the market (EU Regulation 1223/2009, article 11.1).
The responsible person shall provide the product information document in electronic or other format to the competent authority of the member state where the document is stored at the address indicated on its label. The information contained in the product information document shall be provided in a language easily understood by the competent authorities of the Member States (EU Regulation 1223/2009, article 11.3).
Product information files must be kept at a single EU/UK address, even if cosmetic products are sold in more than one EU country. It also means that there can only be one person responsible for each product across the EU - for example, there cannot be one person responsible for the same product in Germany and one in France.
Product Information Document (PIF) content
The contents of the product information document are described in Article 11 of EU Cosmetics Regulation 1223/2009 and Schedule 34 of the UK Product Safety and Metrology (Amendment, Etc.)(EU Withdrawal) Regulation 2019. The content of the cosmetic product information file is standardized and will not change regardless of the type of cosmetic product involved.
In accordance with clause 11, the product information file must contain the following information and data.
Description of cosmetic products
The contents of the Cosmetic Product Safety Report (CPSR) are shown in Annex I, including the following contents.
Cosmetic Safety Information
Quantitative and qualitative components of cosmetics
Physicochemical properties and stability of cosmetics
Microbial quality
Information on impurities, traces, packaging materials
Normal and reasonably foreseeable use
Touch cosmetics
Exposure to these substances
Toxicological profile of the substance
Adverse effects and serious adverse effects
Information about cosmetics
Safety assessment of cosmetic products
Appraisal conclusion
Indicate warnings and instructions
reasoning
Credentials and accreditation of the assessor
Description of manufacturing method and good manufacturing Practice certificate or declaration of compliance with good manufacturing practice (GMP standard which must be followed in the EU is standard ISO 22716) 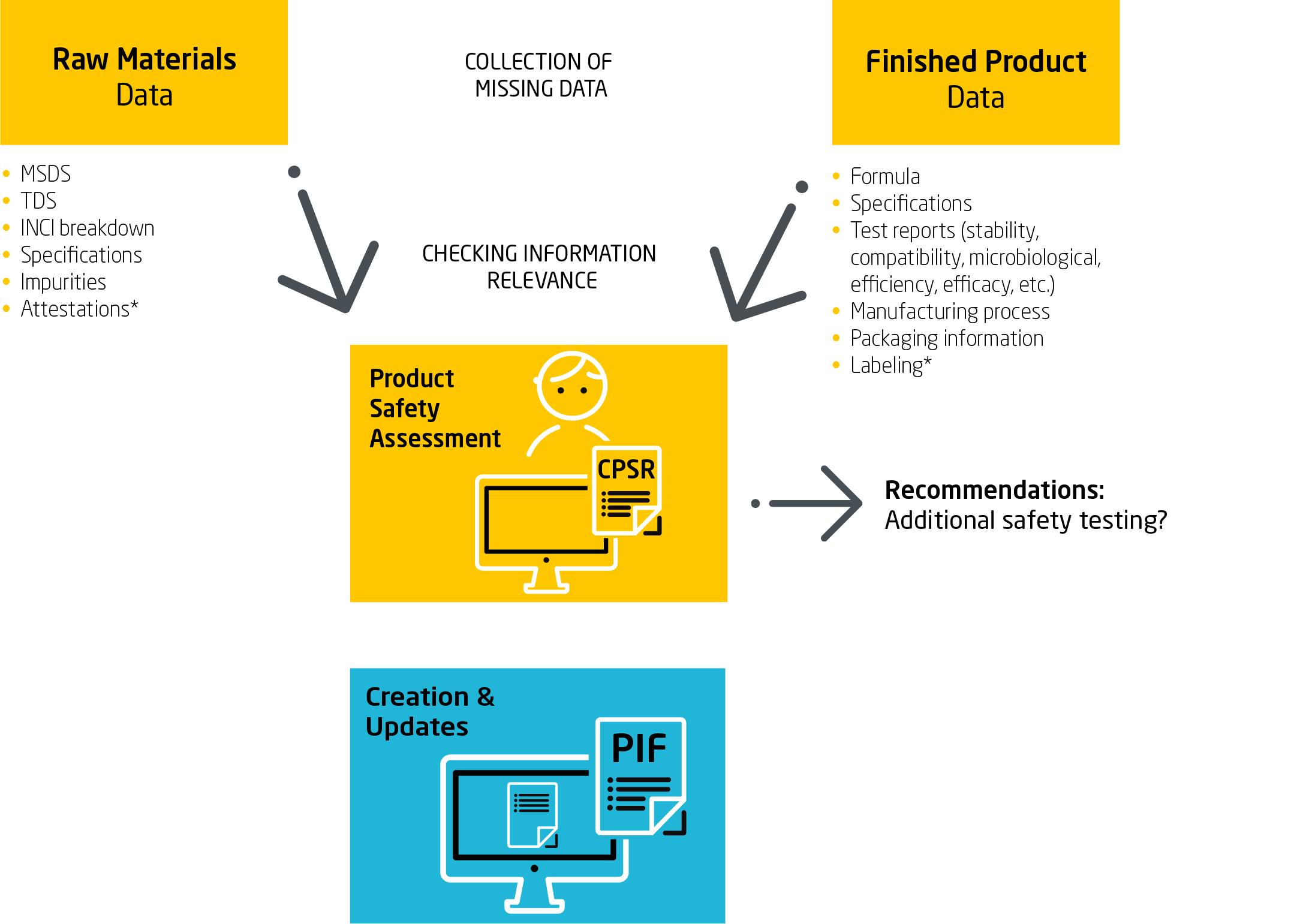
Demonstrate the efficacy of the cosmetic in reasonable circumstances
Data from any animal tests
Product information files should also contain product labels
The most important and extensive part of the Cosmetic product information file is the Cosmetic Product Safety Report (CPSR), which contains all information, specifications and tests related to raw materials, finished products, packaging, etc. Based on these, the safety and compliance of cosmetic products with EU/UK cosmetic regulations can be demonstrated.
The complete Cosmetic Product Information Document (PIF) is the basis for notification to the EU CPNP portal or the UK SCPN Portal and must be implemented before cosmetic products can enter the EU/UK market.